On Monday morning I left a cold and rainy Sydney and boarded a plane to head north to the sunny Gold Coast for an international protein conference.
To be more precise, the 6th General Meeting of the International Proteolysis Society held at the Marriot Hotel in Surfers Paradise. As one can expect in Queensland at this time of year, the weather is warm and very humid (read: lovely). We arrived at the end of a weekend of street car racing, so much of the centre of town is still cordoned off with barriers, fences and large signs for beer companies. Evidence of a recent bogan invasion is rife.

Poolside at the Marriot Hotel, Surfers Paradise. The famous natural reef complete with tropical fish.
Many of the conference delegates are clearly excited to be in such a location, one even stating that it is proof that “proteolysis takes you to paradise!” Suffice to say, there is a definite tropical/resort atmosphere and attitude permeating proceedings.
However, we must not forget this is about the science, and in particular, proteases.
What are proteases?
Essentially they are enzymes, (like the ones you may have heard about on television commercials for washing powder), which are responsible for chewing up and recycling proteins.
If you remember back to high school genetics, you would recall that DNA, which is made up of hundreds of thousands of genes, (about twenty three thousand in the case of the human genome) holds the code for all our proteins. Enzymes are a class of these proteins, assigned many and varied tasks, such as protecting our cells from stress, helping to protect our DNA from damage and recycling damaged proteins.
The proteases (which are also enzymes) have varied roles including turning other proteins on and off and helping our cells to reproduce. But, like many functions in the human body such as the immune system, sometimes things can go wrong and instead of being useful, become toxic and cause harm.
Although we need proteases to survive, they can also contribute to illnesses such as cancer. One particular type of protease known as “cathepsin S” on one hand, helps our immune system fight invading pathogens, but on the other, has been shown to assist in the spread of cancer.
And humans are not the only ones who need proteases to survive. Parasites such a malaria and hookworm (a member of the Helmith family) need them to extract energy from their food. And in the case of these critters, human blood is what they love!
What about Malaria?
Malaria remains a huge global health crisis; 1 child dies every 5 seconds, there are 5 million cases every year of which 2 million die and a further 3 billion people are at risk of infection. The majority of these people are in the developing world and many are children less than 5 years old.
Dr Sheena McGowan is a structural biologist from Monash University in Melbourne who has been studying the proteases of the malarial parasite in a search for new drugs. Her approach focuses on the feeding cycle of the malarial parasite which involves invading red blood cells and feeding on the haemoglobin by breaking it down into single elements. This “breaking down” process requires a protease, and Sheena has figured out if she can block the function of the protease, she could effectively starve the parasite.
In collaboration with a large group of scientists from all across the world, Dr McGowan has been able to determine the precise structure of the malarial protease, using an approach known as X-ray crystallography (see below). This is a very complex and difficult process, but in principle, by obtaining crystals of the protease and firing X-ray beams at it, scientists can work out the shape and precise structure. This information then allows Sheena and her team to design compounds that will block it from working. A bit like putting a key in to a lock, but coating it with superglue first, so that it sticks really tight and never comes out.
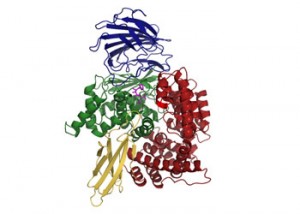
The crystal structure of the malarial digestion enzyme. The drug that may be able to inhibit it ("the key") is shown in magenta.
Dr McGowan’s work will help address the burgeoning problem of malarial infection in the developing world, especially in places where severity of the infection is exacerbated by co-infection with other infectious diseases such as HIV or tuberculosis.
In the meantime there is some good news on the malaria front, with a scheduled roll out of a vaccine across Africa in 2010. But, Dr McGowan is cautious to remind us that this will not be the end of the malaria scourge.
Unfortunately, parasites are clever little buggers, rapidly evolving ways to get around our drugs. This is why work like that of Dr McGowan and her team is so important in tackling new and innovative methods to design new drugs against infectious diseases.







